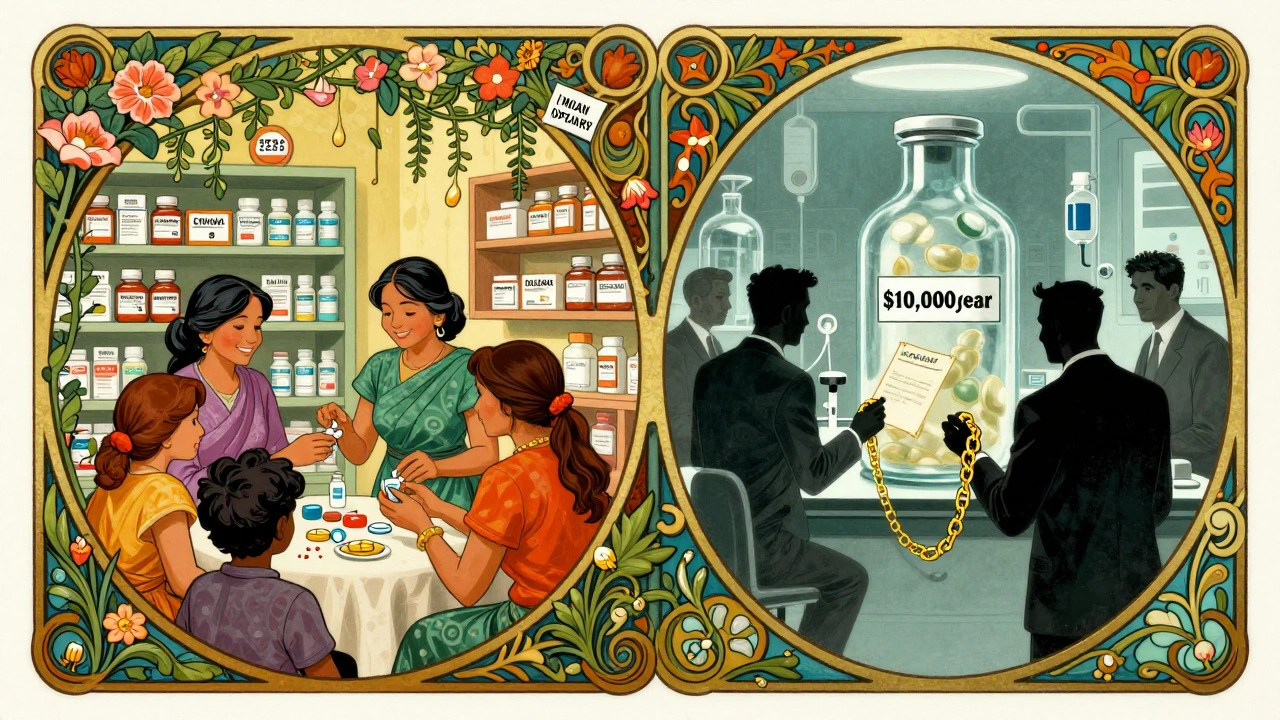
Before the TRIPS Agreement, many developing countries could make cheap generic versions of life-saving drugs. In India, for example, a generic version of the HIV drug AZT cost $1.50 a month. In the U.S., the same drug cost over $10,000 a year. That gap wasn’t because of efficiency-it was because India didn’t recognize product patents on medicines. That changed in 1995, when the TRIPS Agreement came into force. Suddenly, every WTO member had to follow the same patent rules. And for millions of people who rely on generics to survive, that shift made a deadly difference.
What TRIPS Actually Does to Drug Patents
The TRIPS Agreement, part of the World Trade Organization’s rules, forces all 164 member countries to give drug companies at least 20 years of patent protection from the date they file. That might sound fair-until you realize that most of that time passes before the drug even hits the market. Clinical trials, regulatory reviews, and manufacturing setup can take 7 to 10 years. So by the time a drug is approved, companies already have 10 to 13 years left on their monopoly. That’s not innovation protection-it’s extended monopoly.
TRIPS also bans countries from using process patents only. Before TRIPS, India and others could make the same drug using a different manufacturing method. That’s how they kept prices low. TRIPS closed that loophole. Now, if a drug is patented anywhere, you can’t make it-even if you use a different chemical pathway. And it gets worse. TRIPS introduced something called data exclusivity. That means even after a patent expires, generic makers can’t use the original company’s clinical trial data to prove their drug works. They have to run their own expensive trials. That adds 5 to 10 years of extra delay before generics can enter the market.
The Real Cost: Prices, Delays, and Deaths
The numbers don’t lie. A 2001 study in the Journal of the American Medical Association found that after TRIPS, prices for patented drugs in developing countries jumped by over 200%. In South Africa, when they tried to pass a law allowing generic HIV drugs in 1998, 40 pharmaceutical companies sued them. The lawsuit was dropped only after global protests. In Brazil, the government started making its own generic antiretrovirals in 2000. The U.S. threatened trade sanctions. Brazil didn’t back down-and eventually, the U.S. dropped the case.
India, once the pharmacy of the developing world, had to change its patent laws by 2005. The result? Cancer drugs like imatinib (Glivec) went from $2,500 a year to over $10,000. That’s not a price increase-it’s a death sentence for people who can’t pay. A 2008 Lancet Oncology study showed that 80% of patients in India who needed imatinib couldn’t afford it after the patent kicked in. Even today, the Access to Medicine Foundation found that 65% of low-income countries report delays in approving generics because of patent rules that go beyond what TRIPS requires. These are called TRIPS Plus provisions-extra barriers pushed by rich countries through trade deals.
Compulsory Licensing: The Legal Loophole That Almost Never Works
TRIPS does allow one escape hatch: compulsory licensing. This lets governments let generic makers produce a patented drug without the company’s permission-if there’s a public health emergency. Thailand used it for HIV and heart drugs. Brazil used it for HIV treatment. Both faced political pressure. But here’s the catch: TRIPS says the license must be used mostly for the country’s own market. So if you’re a small African nation with no drug factories, you can’t import generics from India-even if India has the capacity to make them.
In 2003, WTO members tried to fix this with the ‘Paragraph 6 Solution.’ It allowed countries without manufacturing capacity to import generics made under compulsory license. Sounds good, right? But the paperwork was so complex, and the legal risks so high, that only two countries ever used it: Canada and Rwanda. In 2009, Rwanda imported 200,000 doses of antiretrovirals from Canada. That was it. No other country has successfully used the system since. The system was designed to help-but it’s so hard to use that it might as well not exist.

TRIPS Plus: The Hidden Rules That Matter More
The real problem isn’t just TRIPS. It’s what comes after it. The U.S., EU, and Switzerland keep pushing stronger patent rules in bilateral trade deals. The EU-Vietnam Free Trade Agreement (2020) gives 8 years of data exclusivity-longer than TRIPS allows. The U.S.-Mexico-Canada Agreement (USMCA) extends patent terms for biologic drugs to 10 years. These aren’t global rules-they’re hidden clauses that only affect countries with less power.
By 2020, 85% of U.S. free trade agreements included TRIPS Plus provisions. That means countries like Kenya, Ghana, or Bangladesh are being forced to give drug companies even more control than TRIPS already does. They can’t refuse. If they want access to U.S. markets or foreign investment, they have to agree. So while TRIPS sets the floor, TRIPS Plus builds the ceiling-and it’s crushing access to medicines.
Who Benefits? Who Gets Left Behind?
Pharmaceutical companies argue that strong patents are necessary to fund innovation. They say 73% of new drugs since 2000 came from companies in strong IP countries. But here’s what they don’t say: 80% of those drugs are for conditions that affect rich people-diabetes, heart disease, erectile dysfunction. Only 13 out of 1,223 new drugs approved between 1975 and 1997 were for tropical diseases like sleeping sickness or Chagas disease. Those diseases kill millions in poor countries. But they don’t make money. So no one invests.
Meanwhile, the global generic medicine market hit $420 billion in 2020. But almost all of that growth happened in rich countries. In low-income nations, 80% of medicines are off-patent-but still unaffordable because of supply chain issues, weak health systems, and patent barriers. The Global Fund found that antiretroviral costs dropped from $10,000 per patient per year in 2000 to $75 in 2019-thanks to generic competition. But that’s only for first-line drugs. Second-line treatments? Still $300 to $600 a year. Most people can’t pay that.

The 2022 TRIPS Waiver: A Small Step Forward
The pandemic forced a reckoning. In October 2020, India and South Africa proposed a temporary waiver of TRIPS protections for COVID-19 vaccines and treatments. Over 100 countries supported it. The U.S., EU, and Switzerland blocked it for over a year. In June 2022, they finally agreed to a limited waiver-but only for vaccines, not treatments. And it only applies to countries with low vaccination rates. It’s not a full solution. But it was the first time WTO members agreed to change TRIPS for public health.
Still, the waiver has no expiration date for use. It’s not automatic. Countries still need to apply. And no one has used it yet. Why? Because the real bottleneck isn’t the law-it’s manufacturing capacity, supply chains, and corporate control over technology. The waiver didn’t force companies to share their formulas. It just removed one legal barrier. That’s not enough.
The Medicines Patent Pool: A Better Way?
One bright spot is the Medicines Patent Pool, created in 2010. It’s a nonprofit that negotiates voluntary licenses with drug companies to let generic makers produce cheaper versions of HIV, hepatitis C, and tuberculosis drugs. As of 2022, it had reached 17.4 million people in low- and middle-income countries. It works because it’s voluntary. Companies get royalties. Generic makers get access. Patients get medicine.
But it’s not a system. It’s a patchwork. It depends on companies agreeing to cooperate. And they don’t always. The Pool couldn’t get licenses for newer COVID-19 antivirals like Paxlovid. Pfizer refused. So the Pool couldn’t help. That’s the problem with relying on goodwill. When profits are involved, goodwill disappears.
What Needs to Change
TRIPS wasn’t designed to save lives. It was designed to protect corporate profits. And it succeeded. But it’s failing humanity. The fix isn’t complicated:
- Remove data exclusivity for all medicines
- Allow cross-border compulsory licensing without restrictions
- End TRIPS Plus provisions in trade deals
- Require patent holders to license essential medicines to generic producers during public health emergencies
- Invest in public-sector R&D for diseases that affect the poor
There’s no reason why a child in Malawi can’t get the same cancer drug as a child in Sweden. The science is the same. The cost of production is the same. The only difference is the law. And laws can be changed.
The question isn’t whether we can afford to fix this. It’s whether we can afford not to.
Does TRIPS ban all generic drugs?
No, TRIPS doesn’t ban generic drugs. It bans countries from making generics while a drug is under patent. But it allows exceptions like compulsory licensing for public health emergencies. The problem isn’t the law-it’s how hard it is to use those exceptions. Many countries lack the legal expertise, political will, or manufacturing capacity to act.
Why can’t poor countries just make their own generics?
Many can’t because they don’t have the factories, regulatory systems, or supply chains. Even if they have the legal right to make generics, they need to build capacity first. And TRIPS doesn’t help them do that. Some countries, like India and Brazil, built strong generic industries before TRIPS. But for countries that didn’t, the rules now make it nearly impossible to start.
Are generic drugs safe?
Yes. Generic drugs must meet the same quality, safety, and effectiveness standards as brand-name drugs. The WHO and U.S. FDA approve them. The difference is cost-not quality. A generic HIV drug made in India is chemically identical to the brand version. The only thing different is the price tag.
What’s the difference between TRIPS and TRIPS Plus?
TRIPS is the minimum standard set by the WTO. TRIPS Plus are extra rules pushed by rich countries in bilateral trade deals-like longer patent terms, data exclusivity, or restrictions on compulsory licensing. These go beyond what TRIPS requires and make it even harder for poor countries to access affordable medicines.
Can a country ignore TRIPS?
Technically, no. All WTO members must comply, or risk trade sanctions. But history shows that when countries stand together-like South Africa did in 2001-they can force change. TRIPS isn’t unbreakable. It’s a political tool. And politics can change.

14 Comments
This is wild but also kinda expected tbh.
I remember when my uncle could buy HIV meds for 500 rupees a month in Pune. Now? He pays 40,000. And they call it progress. My heart aches for the people who can't afford to live.
Honestly? This is why I don't trust big pharma. They're not here to save lives. They're here to cash in. 😔
The TRIPS waiver for COVID vaccines was a tiny crack in the dam - but look how hard they fought to keep it small. Data exclusivity? That’s not innovation protection - it’s corporate extortion. And the fact that only Rwanda and Canada ever used the Paragraph 6 Solution? That’s not a loophole. That’s a trap designed to look like hope.
It is profoundly disconcerting to observe how intellectual property frameworks, ostensibly designed to incentivize innovation, have been perverted into instruments of systemic inequity. The global disparity in access to life-saving therapeutics is not a byproduct of market forces - it is a deliberate architectural outcome of legal regimes that privilege shareholder value over human survival. One cannot help but question the moral calculus of a system wherein a child in Malawi is denied a chemically identical medication solely because of geopolitical power differentials.
India was the pharmacy of the world, and they took that from us. We didn’t just lose profits - we lost dignity. Now every time a kid dies because a pill costs more than a month’s rent, it’s not just a tragedy - it’s a crime. And the worst part? The people who made the rules will never feel it. They’ll sip their espresso in Geneva while we beg for scraps. 🇮🇳💔
You people act like this is new. The West has been exploiting poor countries for centuries. First they stole our resources, now they steal our medicine. And you think a waiver is enough? Pathetic. The entire system is rigged. Wake up.
The data exclusivity provision is not mentioned in the TRIPS Agreement itself - it is an additional barrier introduced through bilateral agreements, which undermines the spirit of the original text.
Let’s be real - if you’re not a billionaire, you’re not supposed to live. The whole system is built to make sure only the rich get the good stuff. And guess what? The rest of us are just collateral damage. But hey, at least the shareholders got their dividends.
TRIPS is just a distraction. The real problem is that developing nations lack the infrastructure to even produce generics - so blaming patents is like blaming a broken lock when the house was never built. Stop crying about the law and start building factories.
This whole thing is a psyop. Big Pharma doesn’t even make most of the drugs - they just own the patents. The real manufacturers are in China and India. And the WHO? They’re in on it. The ‘waiver’? A staged performance to make you feel like change is happening. It’s not. They’re just moving the chess pieces.
So let me get this straight - we spent 30 years forcing the world to adopt a patent regime designed for Silicon Valley startups, then act shocked when it kills people in rural Africa? We didn’t fail to protect innovation - we failed to protect humanity. And now we’re giving ourselves a pat on the back for letting Rwanda import 200,000 doses? That’s not a victory. That’s a funeral pyre with a ribbon tied around it.
I think we need to stop seeing this as a fight between rich and poor. It’s a fight between profit and people. And honestly? The people are winning - slowly, painfully, but they’re winning. The Medicines Patent Pool? That’s real. The fact that India still exports generics to 150 countries? That’s power. We’re not powerless. We just need to keep pushing.
Stop acting like India is some moral hero. We made generics because we stole Western tech. We didn’t innovate - we copied. Now you want us to play by the rules? Fine. But don’t act like we’re saints. The West built the system. We broke it. And now you want us to fix it? No thanks.